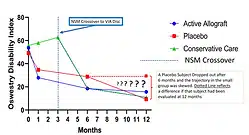
VAST Clinical Trial: Safely Supplementing Tissue Lost to Degenerative Disc Disease

Viable Disc Tissue Allograft Supplementation; One- and Two-level Treatment of Degenerated Intervertebral Discs in Patients with Chronic Discogenic Low Back Pain: One Year Results of the VAST Randomized Controlled Trial

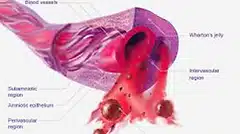
Umbilical cord-derived Wharton’s Jelly may play a role in reducing inflammation and pain and augment healing
Applications of Human Umbilical Cord Blood Cells in Central Nervous System Regeneration
Cord Blood vs BMAC vs Adipose Study
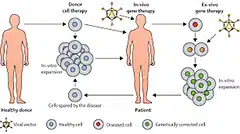
Cell-Based Therapy Using Umbilical Cord Blood for Novel Indications in Regenerative Therapy
Umbilical cord blood-derived mesenchymal stem cells consist of a unique population of progenitors
Potential Uses for Cord Blood Mesenchymal Stem Cells
Umbilical Cord Blood:
A Trustworthy Source of Multipotent Stem Cells for Regenerative Medicine

Treatment of Cerebral Palsy with Stem Cells: A Report of 17 Cases
Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue- Based Products
Umbilical Cord Blood–Derived Stem Cells and Brain Repair
Neural Differentiation and Potential Use of Stem Cells From the Human Umbilical Cord for Central Nervous System

Regulatory Considerations for Human Cells, Tissues, and Cellular and Tissue- Based Products
Comparative Analysis of Secretome of Human Umbilical Cord and Bone Marrow Derived Multipotent Mesenchymal

